With QbD (Quality by Design) as concept, stable and high yield process is achieved by identifying critical process parameters (CPP) based on CQA (critical quality attribute) using DoE experimental process design.
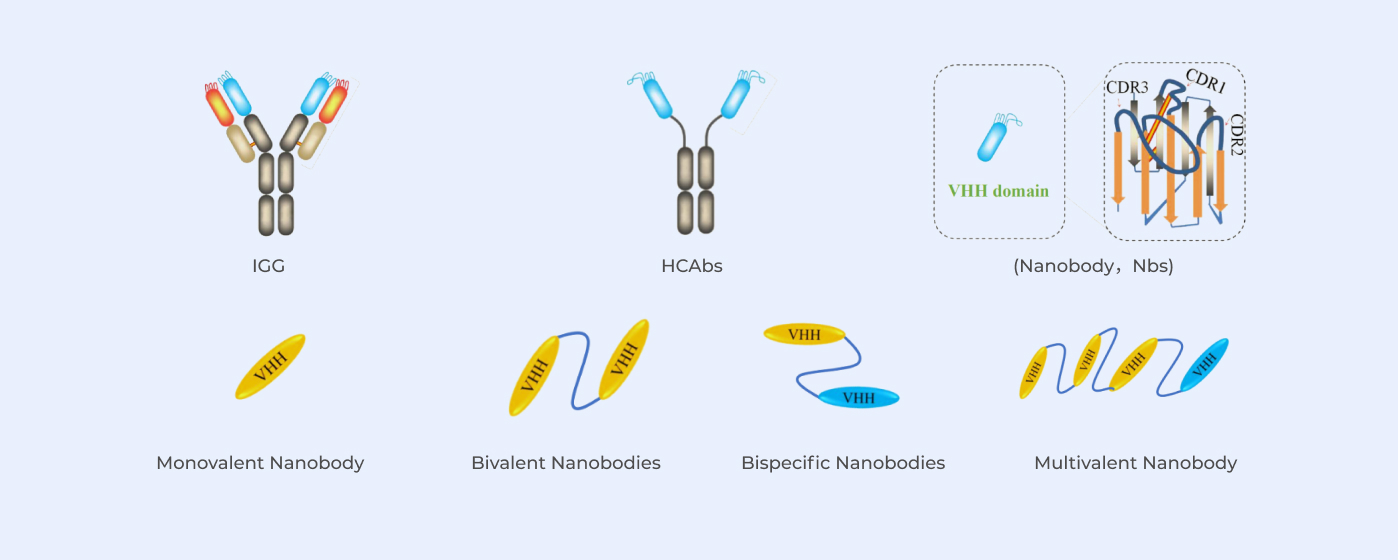 Photo source: International Journal of Nanomedicine 2016:11 3287-3303
Photo source: International Journal of Nanomedicine 2016:11 3287-3303 







With QbD (Quality by Design) as concept, stable and high yield process is achieved by identifying critical process parameters (CPP) based on CQA (critical quality attribute) using DoE experimental process design.

Purity ≥ 95%; endotoxin <50 EU/mg of protein.
Affinity chromatography: after affinity with A3, purity reached 94.1%
Anion exchange chromatography: after the use of 50HQ, yield reached 73.9% and purity reached 98.1%
TEL: +86-523-86285566
Address: Building 29, No. 801, Jiankang Avenue, Taizhou, Jiangsu
Email: BD@yaohaibio.cn
Copyright © Yaohaibio - All Rights Reserved Web design
Privacy policy | Terms & Conditions | Modern Slavery Act Statement | Sitemap